SUNDAY, 22 MAY 2011
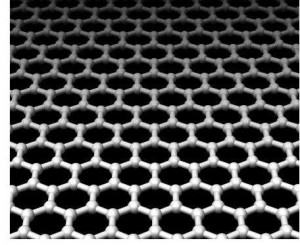 A group at the University of Texas in Austin were trying to make activated carbon with a standard method involving potassium hydroxide. Biomass is the usual carbon source for this process in industry, but the Austin group used graphitic carbon instead, with surprising results. Rather than produce a simple structure full of holes, they instead found that dilute amounts of the potassium hydroxide had restructured the carbon into a three-dimensional network with a very high surface area [1].
A group at the University of Texas in Austin were trying to make activated carbon with a standard method involving potassium hydroxide. Biomass is the usual carbon source for this process in industry, but the Austin group used graphitic carbon instead, with surprising results. Rather than produce a simple structure full of holes, they instead found that dilute amounts of the potassium hydroxide had restructured the carbon into a three-dimensional network with a very high surface area [1].Dr Rodney Ruoff and his colleagues tested their carbon material for use as a supercapacitor and found that it performed surprisingly well, particularly with ionic liquid electrolytes which function over a much larger voltage range. These properties mean that capacitors made from this new carbon material could charge the same amount of energy much faster than a standard lead-acid battery. The method of making these porous carbon materials is also cost-effective and would be straightforward to commercialise, and has the additional potential to be modified to form other three-dimensional graphene-based structures [2].
Written by Stephanie Boardman
References:
- Zhu, Y., Murali, S., Stoller, M.D., Ganesh, K.J., Cai, W., Ferreira, P.J., Pirkle, A., Wallace, A.M., Cychosz, K.A., Thommes, M., Su, D., Stach, E.A. & Ruoff, R.S. 2011. Carbon-based supercapacitors produced by activation of graphene. Science 2011, DOI: 10.1126/science.1200770
- http://www.rsc.org/chemistryworld/News/2011/May/13051102.asp
