THURSDAY, 1 OCTOBER 2009
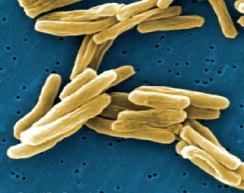
Tuberculosis is a continuing public health threat and is close to becoming a global emergency. The respiratory and infectious disease is most commonly seen in people with lowered immunity and is easily transmitted through the air from person to person during close contact. In most cases of tuberculosis (TB) the inhaled bacteria infect the lungs, although they can spread to other parts of the body. Tuberculosis is treated with long courses of antibiotics which usually last for many months or even years. Although this is an effective therapy, the prevalence of TB is rising because patients do not always complete their prescribed treatment.Three million die from TB annually and each year more than 400,000 new cases with antibiotic resistance are diagnosed. These new drug resistant forms of the bacteria generally stem from poor patient compliance, where sufferers do not follow the required course of treatment, compounded by a long period where the patient often feels better before completing their treatment and stops taking the drugs. Ensuring patients complete a full course of treatment is critical to preventing the outbreak of new drug resistant strains.
The reasons for non-compliance amongst TB sufferers vary. Patients find it troublesome to take a combination of pills and as symptoms improve, they may forget or avoid taking their drugs. Furthermore, some patients do not ever feel physically unwell.
Current treatments for TB are only effective when patients take their medication as instructed. To address compliance issues, the World Health Organization has established a control program to monitor and evaluate the disease regularly; this is known as Directly Observed Treatment, Short-course, or DOTS. Strategies such as DOTS aim to assure patient compliance and avoid drug resistance, yet problems remain. Regular monitoring proves difficult in areas where the availability of healthcare staff is limited or patients need to travel long distances to clinics. Those working at the community level are still seeking an assured way of increasing compliance in order to reduce the number of patients developing resistance and ultimately improving the overall results of tuberculosis control programmes. To optimise the measures taken by the WHO, Inderm plans to collaborate with existing programs such as DOTS.
In order to address this public health problem, Inderm, a start-up company founded by four Cambridge students, is developing a drug-delivery device for TB. Inderm addresses the need for a solution that enforces therapeutic compliance by creating a biodegradable, subdermal drug-delivery device that can release the appropriate therapy over the full treatment period. The system will release the required drugs at a controlled rate, assuring proper treatment of affected patients at a target cost competitive with current treatment costs.
Since significant non-compliance becomes an issue once patients begin to feel better, the Cambridge team is focusing on patients during the last four months of treatment. Currently Inderm is working to raise funds to continue developing their prototype device. The work is focused on the drug-release profile and patient attributes, assuring patient safety, effectiveness and acceptance. The goal is that Inderm can store and release the TB drugs in a way that matches the therapeutic profile, without degradation, toxic accumulations, irregularities in the release profile or negative interactions with the delivery system or the patient.
Sahil Kirpekar and Ali Ansary recently completed an MPhil in Bioscience Enterprise at the Department of Biotechnology and Chemical Engineering