SUNDAY, 5 MARCH 2017
The theory of evolution is up there with the ‘universal law of gravitation’ and the ‘theory of general relativity’ when it comes to popular science. Darwin and Lamarck’s famous historical showdown is ensconced in GCSE Science textbooks, with Darwin emerging as the heroic victor, whilst Lamarck’s work is relegated to history’s dumping ground for scientific theories that were proved wrong. At first glance, it appears that the debate is settled: evolution occurs through natural selection rather than the inheritance of acquired characteristics. Or so we are told.It is perhaps not surprising that the matter was a lot more complex than the textbooks portray. Only decades after Darwin’s death, the Austrian scientist Paul Kammerer produced evidence seemingly in support of Lamarckian inheritance. By forcing midwife toads, which usually mate on land to instead do so in water, he bred toads that appeared to have black ‘nuptial pads’ on their feet. Since these spiked swellings helped males to grasp onto females during mating, their heritable acquisition seemed to be of obvious advantage. It was a discovery that would have turned the evolutionary theory on its head… Except for the accusations that emerged soon afterwards suggesting that the nuptial pads had been faked with black ink. Kammerer’s subsequent suicide, interpreted by many as an admission of guilt, seemed to settle this. However, in 2009 fresh controversy once again emerged when Alexander Vargas argued that Kammerer’s results may in fact have been authentic, explainable by our modern understanding of epigenetics. Was Kammerer the tragically wronged ‘father of epigenetics’ or just another lesson against scientific misconduct? Perhaps we shall never know.
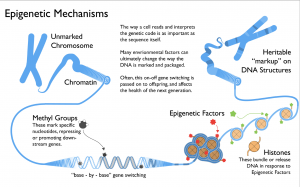 Image: Oran Maguire.
Image: Oran Maguire.With the explosion of research in this field in recent years, effects of epigenetics have been discovered that reach into all aspects of human life, including both health and disease. Epigenetic changes control the differential expression of genes that we inherit from our mother and our father, known as genetic imprinting. Environmental factors, such as diet have been reported to affect DNA methylation patterns. So called ‘epimutations’ have been implicated in several cancers, to give but a few examples.
However, the transmission of epigenetic markers across generations is much less well understood. A landmark study involved the ‘Dutch Hunger Winter’ of 1944-45, when a German blockade exacerbated food shortages in a country which had already been devastated by four long years of war. Women who were pregnant in their third trimester during this famine were more likely to have children at risk of diabetes and grandchildren who were obese. Interestingly, this was because epigenetic imprints were established on the reproductive cells during this stage of pregnancy and so the effects were more dramatic on the second generation more than the first. The theory is that these changes would have improved the well-being and survival of the child if its environmental conditions had remained constant, but a post-war period of plentiful food meant that this epigenetic reprogramming actually became maladaptive and increased susceptibility to disease. Perhaps more shockingly, some of these effects seemed to carry over into the grandchildren of those affected mothers, although data for this is still lacking since the average age of this generation is still relatively low for the study of adult-onset diseases.
"In mice, long-term social instability in adolescence can alter social interaction across as many as three generations"
Admittedly this is an extreme example, but there is also evidence that acquired behaviours can be passed down epigenetically. We have all heard that our early experiences can shape our psychological makeup, but is it possible that some of these effects could be passed on to our children? So far most evidence in favour of this comes from animal models, but is nevertheless extremely shocking. In mice, long-term social instability in adolescence can alter social interaction across as many as three generations. Some mice were seemingly asymptomatic, but nonetheless transmitted the effect to their offspring. Similarly, traumatic experiences in newborn mice can lead to depressive behaviours in later life, again transmitted down up to three generations. Comparable evidence exists for the ability to cope with stress, addictive behaviours (which are already known to run in human families) and cognitive functions. The possible implications for human diseases are profound - just imagine if all of those essay crises could be affecting your future children! (Of course, this is purely fanciful, since inheritance is likely determined by the duration and severity of the experience, but even so, it is a scary thought.)
These behavioural studies are also supported by evidence on a molecular level. Changes in DNA methylation patterns within the egg and sperm have been found in mice exposed to traumatic stress, and these have been traced through several generations. How this occurs is very much an area of active research, especially since it is generally thought that most epigenetic marks are reprogrammed after fertilisation.
Epigenetics is a dynamic field still shrouded in much mystery. There are yet a number of unanswered questions – for example, we don’t know how the effects we see in mice might translate to humans, or indeed if the epigenetic modifications we have identified are truly responsible for the effects observed. However, understanding epigenetic mechanisms could be invaluable to understanding the development of diseases and applying this knowledge to design new treatments. On the other hand, acquired epigenetic modifications can be adaptive, providing a survival advantage that is benefited by natural selection. Perhaps then, we are moving forward from the twentieth century era of genetic determinants of evolution towards a new unified theory of evolution: one that reconciles the historical views of Lamarck with those of Darwin, allowing us to integrate genetic and environmental factors in a more accurate understanding of disease causation.
