TUESDAY, 9 APRIL 2013
The hormone that is making you hungry might also be making you smarter.There is growing evidence of an important relationship between metabolic processes and cognitive function. For example, caloric restriction, the dietary regimen that limits calorie intake, has been shown to reduce age-related cognitive decline in humans and several animal models, while also augmenting the production of new brain cells in a process referred to as ‘neurogenesis.’ The mechanisms underlying this relationship are not well understood; however, there is evidence that elevated levels of the hormone ghrelin may be responsible.
Ghrelin is a peptide hormone synthesised primarily in the stomach and released into the blood stream in response to a negative energy state. As ghrelin acts to regulate energy homeostasis, appetite, body weight and adiposity, researchers predominately viewed it as a potential target for anti-obesity treatments. Whilst the pharmacological treatments and vaccines developed to block or neutralise ghrelin actions have been largely unsuccessful at treating obesity due to the existence of compensatory mechanisms in the body, this potent appetite stimulant holds broad therapeutic potential because of its cognitive-enhancing properties. In particular, there is a growing literature suggesting that a disruption in the normal modulation of ghrelin secretion may contribute to both the metabolic changes and cognitive impairments associated with age-related neurodegenerative diseases.
The non-metabolic actions of ghrelin, such as its pro-cognitive, antidepressant and neuroprotective properties, have only begun to receive attention from the scientific community relatively recently. Studies using rodents have repeatedly demonstrated that ghrelin treatment, administered directly into the brain or via systemic injections, improves memory in a dose-dependent manner. Ghrelin treatments have been shown to improve performance on cognitive tasks measuring spatial memory tested in a maze, recognition memory using repeated presentations of objects, and inhibitory-avoidance learning where rodents avoid a previously aversive stimulus. The molecular mechanism underpinning these robust effects on cognition are not clearly understood; however, it suggests that ghrelin holds potential as a viable target for pharmacological interventions aimed at improving cognitive function in humans.
Along with enhancing cognition, ghrelin treatments appear to cause positive physical changes in the brain, such as increasing neurogenesis in an area of the brain called the ‘hippocampus,’ which is a structure critically important for memory. It was not until the 1980s that the scientific community acknowledged that a select few regions of the adult brain, including the hippocampus, retain the ability to produce new brain cells called neurons—a process referred to as ‘adult neurogenesis.’ The survival and death of these new neurons appear to be input-dependent and are affected by experience. Although the function of adult neurogenesis remains largely unknown and debated, evidence suggests that these new cells make distinct contributions to learning and memory. Not only does increasing the amount of circulating ghrelin stimulate the production of new neurons in an adult rodent’s brain, but these new neurons may underlie ghrelin-dependent improvements in cognition. In addition to promoting cell proliferation, ghrelin is also neuroprotective. Both in cell cultures (in vitro) and in living organisms (in vivo), ghrelin protects neurons when exposed to stressors. Thus, ghrelin increases the number of neurons by both stimulating neurogenesis and preventing cell death.
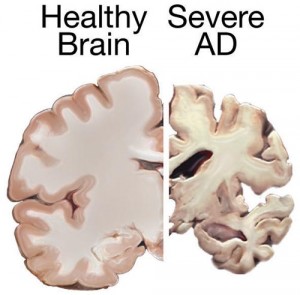
The implications of this research are far reaching. In particular, the pro-cognitive and neuroprotective properties of ghrelin have potential clinical applications for Alzheimer’s disease. Alzheimer’s is a devastating neurodegenerative disease, making it a major public health concern as the elderly population continues to increase worldwide. Unfortunately, efforts towards developing effective treatments have shown limited efficacy. There are several converging lines of evidence linking metabolic syndromes with an increased risk of developing Alzheimer’s. For example, patients suffering from neurodegenerative disorders often display coexisting metabolic dysfunction. In fact, some refer to Alzheimer’s as “type 3 diabetes”, reflecting one theory that it represents a form of diabetes that selectively involves the brain. There is a growing literature suggesting that insulin deficiency and insulin resistance act as mediators of Alzheimer’s-type neurodegeneration. As ghrelin has been shown to modulate insulin sensitivity, as well as several other metabolic and mnemonic effects, it may be a candidate molecule responsible for the relationship between metabolic and cognitive dysfunction.
Indeed, there is increasing evidence suggesting an association between ghrelin and Alzheimer’s pathology. The first line of evidence is that involuntary weight loss and nutritional deficiencies are common in individuals diagnosed with Alzheimer’s, as well as in the cognitively-impaired, non-demented elderly. Since ghrelin is an important regulator of appetite, age-related weight loss supports the reported age-related decline of ghrelin concentrations. Importantly, weight loss appears to precede cognitive impairment in patients with Alzheimer’s, suggesting that metabolic changes could be targets for early detection and prevention of cognitive decline.
A second line of evidence in support of a role of ghrelin in the development of Alzheimer’s comes from animal models. In at least two mouse models of Alzheimer’s, ghrelin treatment has been shown to rescue performance on tasks measuring memory retention, while also attenuating Alzheimer’s -associated neuropathological abnormalities. Furthermore, neurons treated with ghrelin for one hour show decreased tau hyperphosphorylation, which is one of the hallmarks of Alzheimer’s pathology.
More research is needed before any conclusions can be drawn about the role of ghrelin in the development of Alzheimer’s. However, ghrelin plays a central role in metabolic functions—dysfunction of which appears to be closely linked with Alzheimer’s pathology—while also directly stimulating the higher brain functions most affected by the disease, such as learning and memory. Understanding how ghrelin acts in the brain holds promise for new therapies. Importantly, one of the major benefits of ghrelin is that it can be administered systemically and still affect the brain. This discovery will ease the translation of pharmacological interventions from animal models to human patients.
Ghrelin holds the unique and important role of connecting metabolic processes with cognition. From an evolutionary perspective, a link between hunger and memory is beneficial. Ghrelin initiates a search for food by stimulating appetite, but it may also facilitate success by aiding memory for previously discovered food sources. Although the discovery of ghrelin has not yet yielded a miracle diet pill as some had once hoped, there is now strong evidence that it may provide therapeutic targets to protect against cognitive decline. This work also highlights the importance of avoiding an overindulgent diet. Restricting calorie intake is one natural lifestyle change that can increase ghrelin levels and positively impact cognitive processes. So the next time you are reaching for that high calorie snack, do yourself a favor and remember to limit your consumption. Let ghrelin grow your brain and not your waistline.
Brianne is a second year PhD student in the Department of Psychology.
If you would like to learn more about the links between ghrelin and cognitive development, check out these recent papers from Nature Neuroscience and PNAS.